Cell-free DNA Controls (cfDNA)cfDNA Reference Standards are highly characterized, biologically relevant materials used to assess the performance of cfDNA assays that detect somatic mutations via PCR, Next Generation Sequencing (NGS), or other novel readout methods. |
 |
| Part Number | Description |
| Normal female cfgDNA standards |
|
| 0860005 | 1ug in TE buffer |
| 0860006 | 250ng spiked in 5ml plasma, wildtype background |
| Multiplexed ctDNA fragments (~150bp) mixed with nucleosomally fragmented WT cfDNA background in human plasma |
|
| 0860001 | 1ug in TE buffer, 3% AF |
| 0860003 | 125ng in 5ml plasma, 3% AF |
Need a custom variant or a combination of variants? Contact us for your custom variant solutions today!
Gene List
Cell-Derived Variants |
|||
| APC Q1429 | EGFR L858R | KRAS G12C | PTEN R130G |
| APC R1450 | EGFR D770_N771insG | KRAS G12D | TP53 R175H |
| BRAF V600E | EGFR T790M | KRAS G12V | TP53 R248Q |
| CTNNB1 T41A | FGFR3 S249C | NRAS Q61R | |
| EGFR C797S | IDH1 R132H | PIK3CA E545K | |
| EGFR E746-A750del | KIT D816V | PIK3CA H1047R | |
Synthetic Variants |
|||
| AKT1 E17K | HRAS Q61R | NF1 I679Dfs 21 | CCDC6-RET fusion |
| ATM R337C | KIT W557_K558del | NOTCH1 H1601L | CD74-ROS1 fusion |
| CDKN2A R80 | MAP2K1 P124S | PDGFRA D842V | EML4-ALK v1 fusion |
| DDR2 R709 | MDM2 R189H | POLE P286R | ETV6-NTRK3 fusion |
| EGFR G719A | MDM4 Q118L | SMARCA4 T910M | K1F5B-BICC1 fusion |
| ERBB2 S310F | MED12 G44D | STK11 F354L | KIF5B-RET fusion |
| ERBB4 R711C | MET Y1253D | TPS3 R273H | SLC34A2-ROS1 fusion |
| ESR1 D538G | |||
cfDNA Controls for Non-Invasive Prenatal Testing (NIPT)
ZeptoMetrix offers patient-like cfDNA reference materials for Trisomy 13, 18, and 21 for assay development, validation, and routine run QC for NIPT assays.
| Part Number | Description |
| TE-based, nucleosomally fragmented cfDNA product, designed for counting based NIPT assays. | |
| 0860008 | Trisomy 13 Standard for counting based assays, 5% fetal fraction, 1ug in TE |
| 0860009 | Trisomy 18 Standard for counting based assays, 5% fetal fraction, 1ug in TE |
| 0860010 | Trisomy 21 Standard for counting based assays, 5% fetal fraction, 1ug in TE |
| 0860011 | Trisomy 13, 18 & 21 Standard for counting based assays, 5% fetal fraction, 1ug in TE |
| Human plasma-based, nucleosomally fragmented cfDNA product, designed for counting based NIPT assays. | |
| 0860012 | Trisomy 13 Standard for counting based assays, 5% fetal fraction, 125ng in 5mL plasma |
| 0860013 | Trisomy 18 Standard for counting based assays, 5% fetal fraction, 125ng in 5mL plasma |
| 0860014 | Trisomy 21 Standard for counting based assays, 5% fetal fraction, 125ng in 5mL plasma |
| 0860015 | Trisomy 13, 18 & 21 Standard for counting based assays, 5% fetal fraction, 125ng in 5mL plasma |
Product Specifications |
|
| Human plasma | Yes |
| Cell derived cfDNA or synthetic | Cell derived + synthetic (gBlock like) |
| Single background wildtype cells | Yes |
| Fragmentation method | Enzymatic (or sonication) |
| AF % by ddPCR for all mutations | Yes |
| ddPCR value with standard deviation | Yes |
| AF % by NGS with standard deviation | Yes |
| Certification/Compliance | ISO 13485 |
| Intended Use | Simulate human samples to verify and validate |
| Certificate of Analysis | Available upon request |
Why use cfDNA controls and reagents?
Human plasma is a complicated matrix and cfDNA is often present at very low levels. ZeptoMetrix delivers high sensitivity for detection of rare cfDNA presence through unique standards and controls that mimic human specimens.
Systemic lack of TRUE standards and controls results in:
- Poor clinical sensitivity
- Discordance among assays - despite enhancement in sequencing detection, there still remain rampant discordance among liquid biopsy assays. Assays developed in 2019 are no more sensitive than assays developed in 2017.
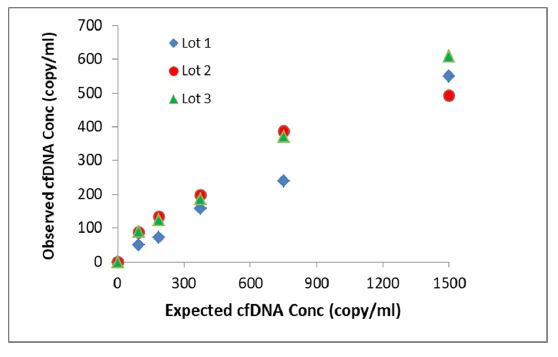
| DNA extraction recovery from different plasma lots |
|